The extracellular matrix (ECM) is a fundamental component in cell biology. The ECM is essential for maintaining the structural and biochemical integrity of any tissue. This complex network of proteins and polysaccharides is dynamic and experiences continuous remodeling depending on various physiological and pathological stimuli.
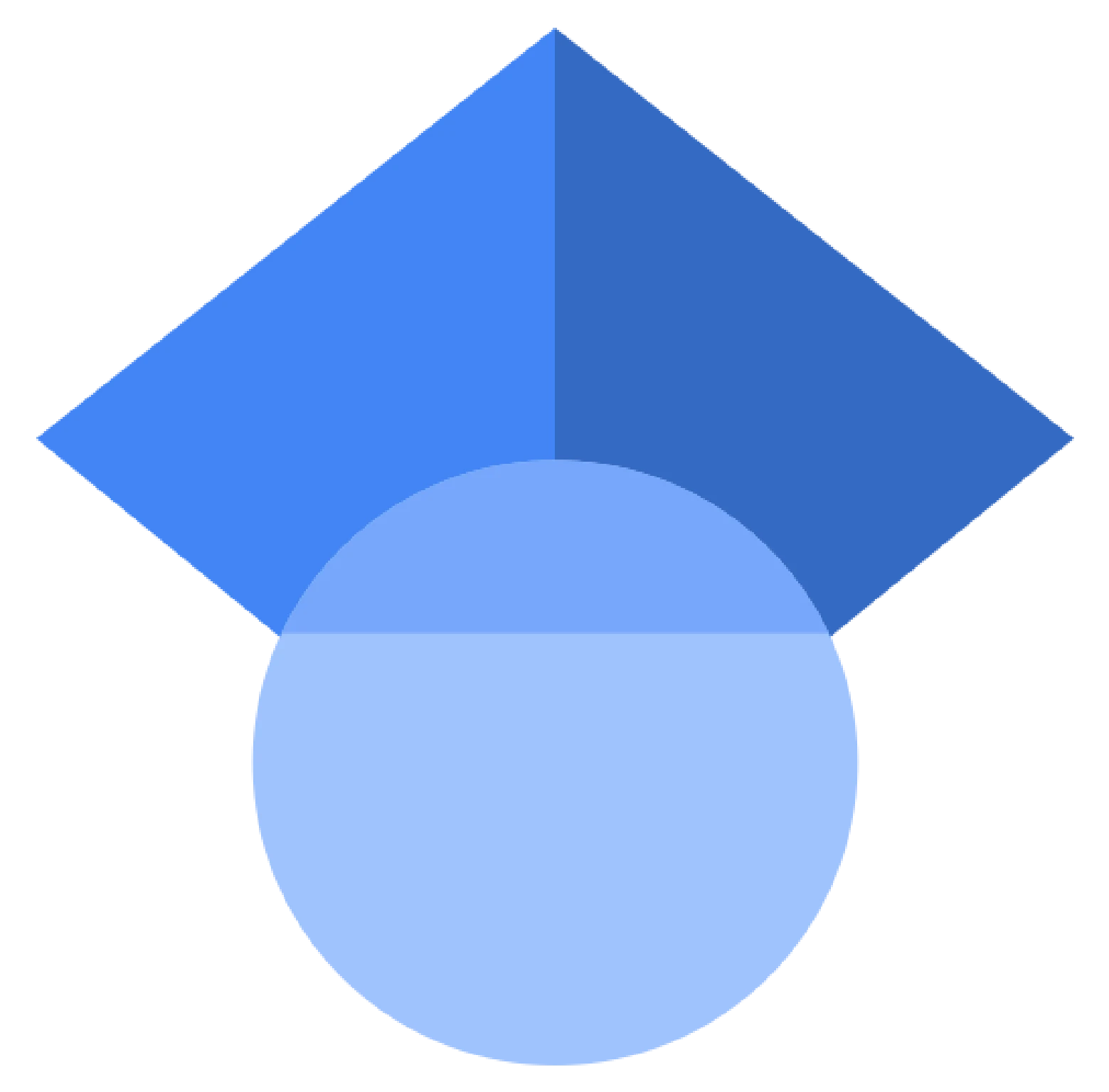
The interphotoreceptor matrix (IPM) is a specialized ECM that envelops the photoreceptor cells in the retina and is essential for retinal health and function. It supports structural stability, facilitates nutrient and waste exchange, and mediates crucial biochemical and biomechanical signals necessary for retinal development, immune responses, and disease progression. Our research is dedicated to decoding the complex roles of the IPM, Its composition, and synthesis. We endeavor to elucidate how the IPM contributes to the progression of visual impairments such as diabetic retinopathy, macular degeneration, and retinitis pigmentosa. By understanding these mechanisms, we aim to innovate therapeutic approaches to combat these conditions.
Our laboratory utilizes advanced techniques and methodologies, including genetically modified mice models, high-resolution microscopy, optical coherence tomography, electrophysiology, adenovirus-mediated gene expression in mice, and various biochemical techniques to analyze protein interactions. We also employ cutting-edge tools such as proteomics, RNA sequencing, and metabolomics to explore the role of IPM in the retina.
Our research, besides its potential to cure blinding diseases, has a broad impact on diverse scientific fields such as aging, neurodegeneration, biomaterials, brain-machine interface, 3D tissue culture, and computational neuroscience.
Areas of interest
Our studies are centered on two important proteins conserved across species and linked to human diseases. These proteins are known as "interphotoreceptor matrix proteoglycans 1 and 2" (IMPG1 and IMPG2). They are proteoglycans rich in glycosylation and chondroitin sulfate. Rods and cones photoreceptors synthesize these proteins in the inner segments (IS). We aim to answer several questions related to these proteins such as: How do IMPG1 and IMPG2 molecules combine to form an IMPG-matrix that surrounds the photoreceptors? Why do IMPG1 and IMPG2 require each other to develop a healthy IPM? What is the exact size and shape of the IMPG-matrix?
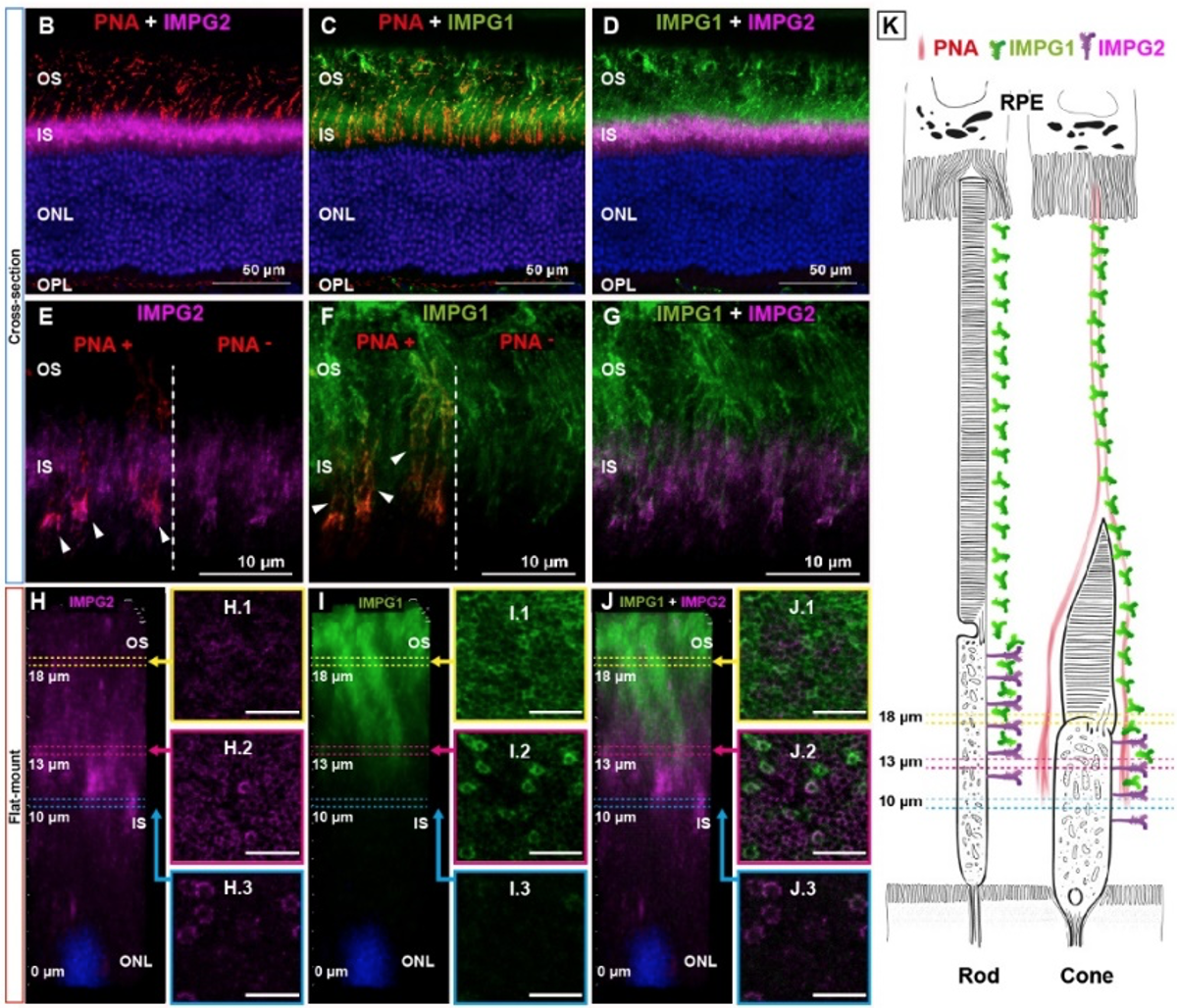
3D reconstruction of a single cone photoreceptor in wild-type retina flat-mount, stained with PNA (red), IMPG1 (green), IMPG2 (magenta), and DAPI (blue).
3D reconstruction of the flat-mount retina from wild-type, IMPG1, and IMPG2 KO mice stained with PNA (red), IMPG1 (green), Gat2 (magenta), and DAPI (blue).

The exchange of nutrients and molecules between the photoreceptors and the RPE takes place through the IPM. Our goal is to understand the role of the IPM in the diffusion of molecules within the photoreceptors-IPM-RPE complex in health and disease. Some of our questions extend to understand if the IPM aging alters its functional properties and how the IPM contributes to the disease mechanism of blinding diseases.
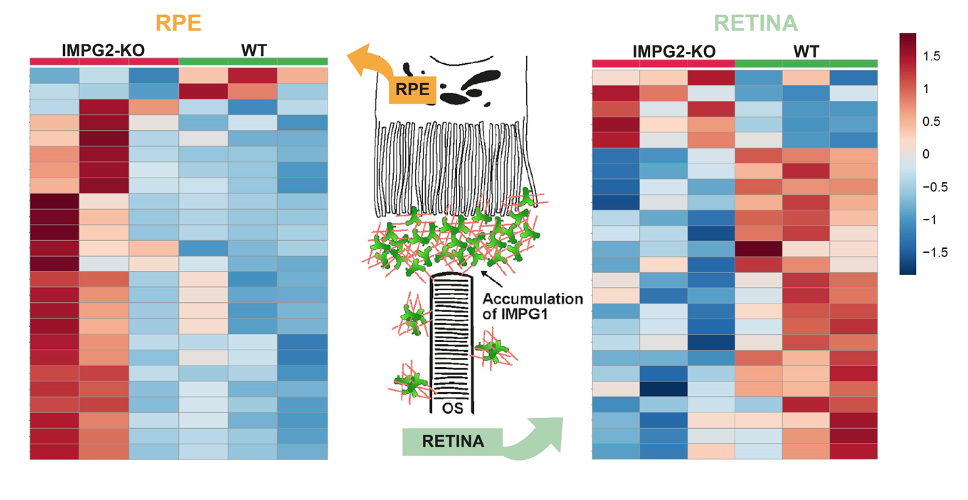
Metabolomics studies have revealed metabolic changes in the retina and RPE of mice with an affected interphotoreceptor matrix.
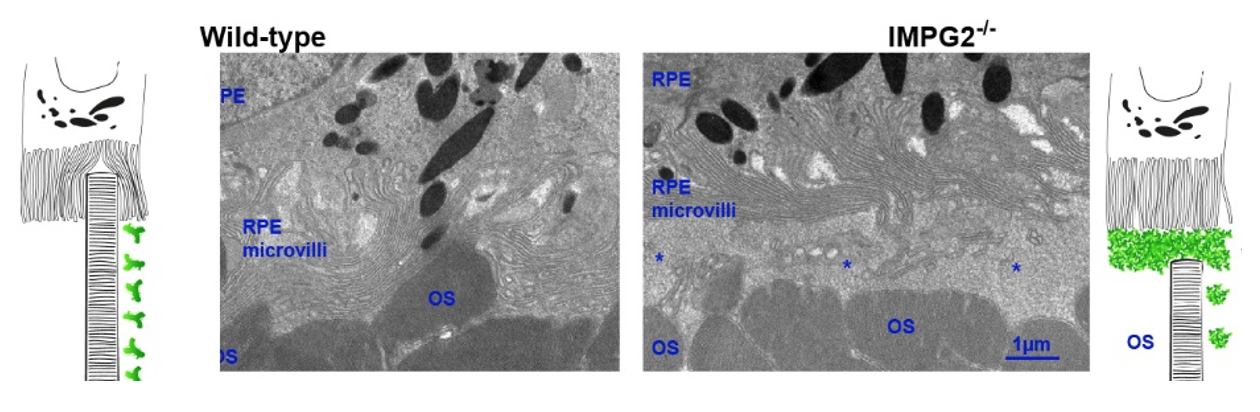
Electron microscopy revealed changes in the extracellular matrix of mutant mice.
Mutations in IMPG1 or IMPG2 genes can cause blinding conditions such as subretinal lesions or retinitis pigmentosa. We are developing adenovirus-based gene therapies to ameliorate or cure these genetic defects. Our goal is to evaluate the efficacy of these therapies in treating IMPG-related pathologies using mouse models that reproduce the genetic defects found in humans.
In addition, we are investigating drugs targeting the IPM as a potential therapeutic for ocular diseases.
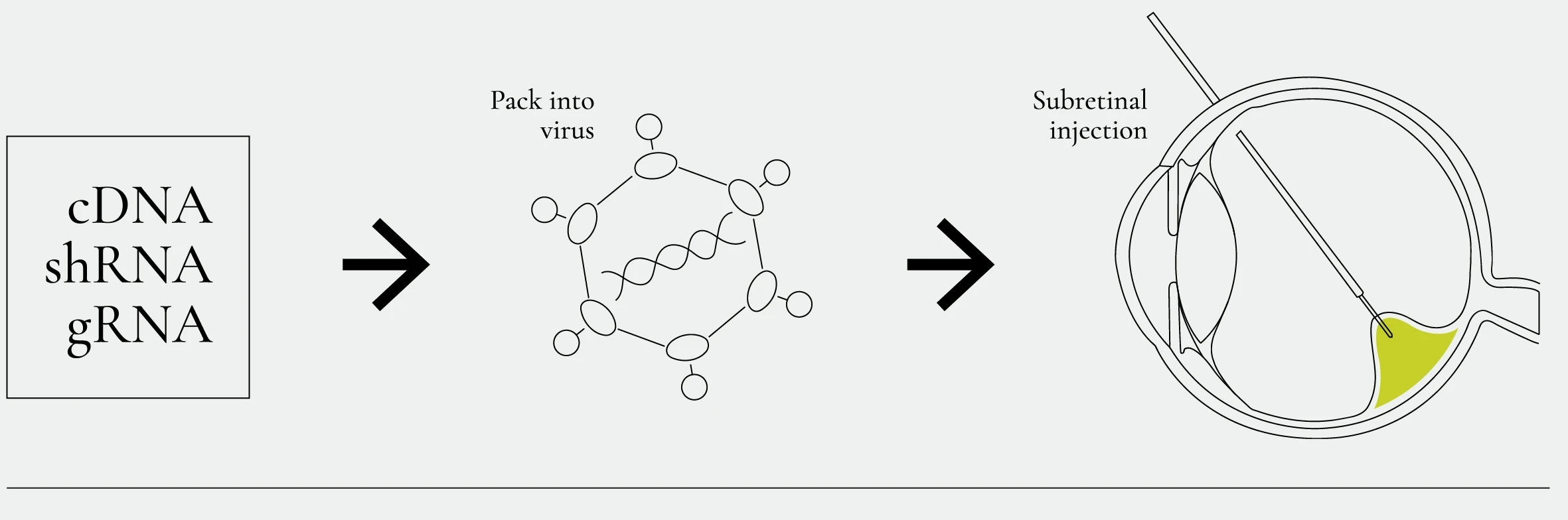
Gene therapy workflow